MULTIVAC - Flexible Packaging Solutions for Medical Products
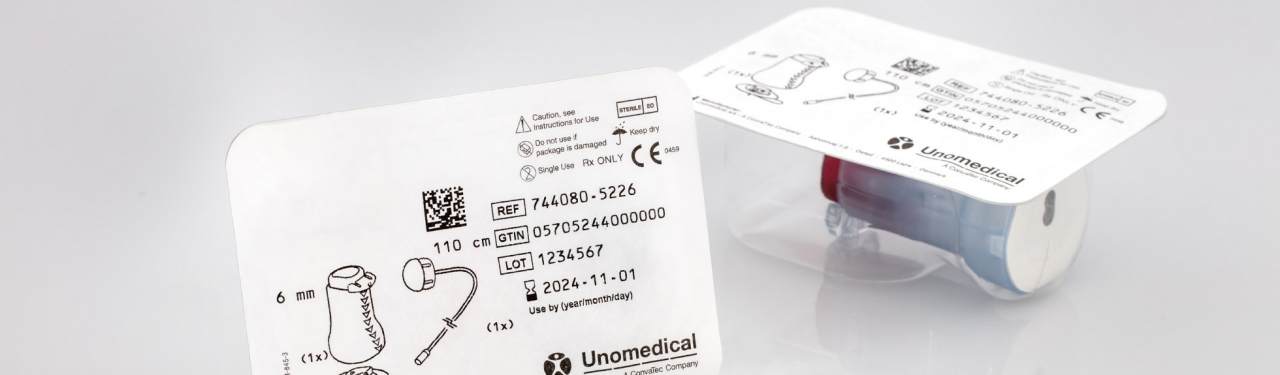
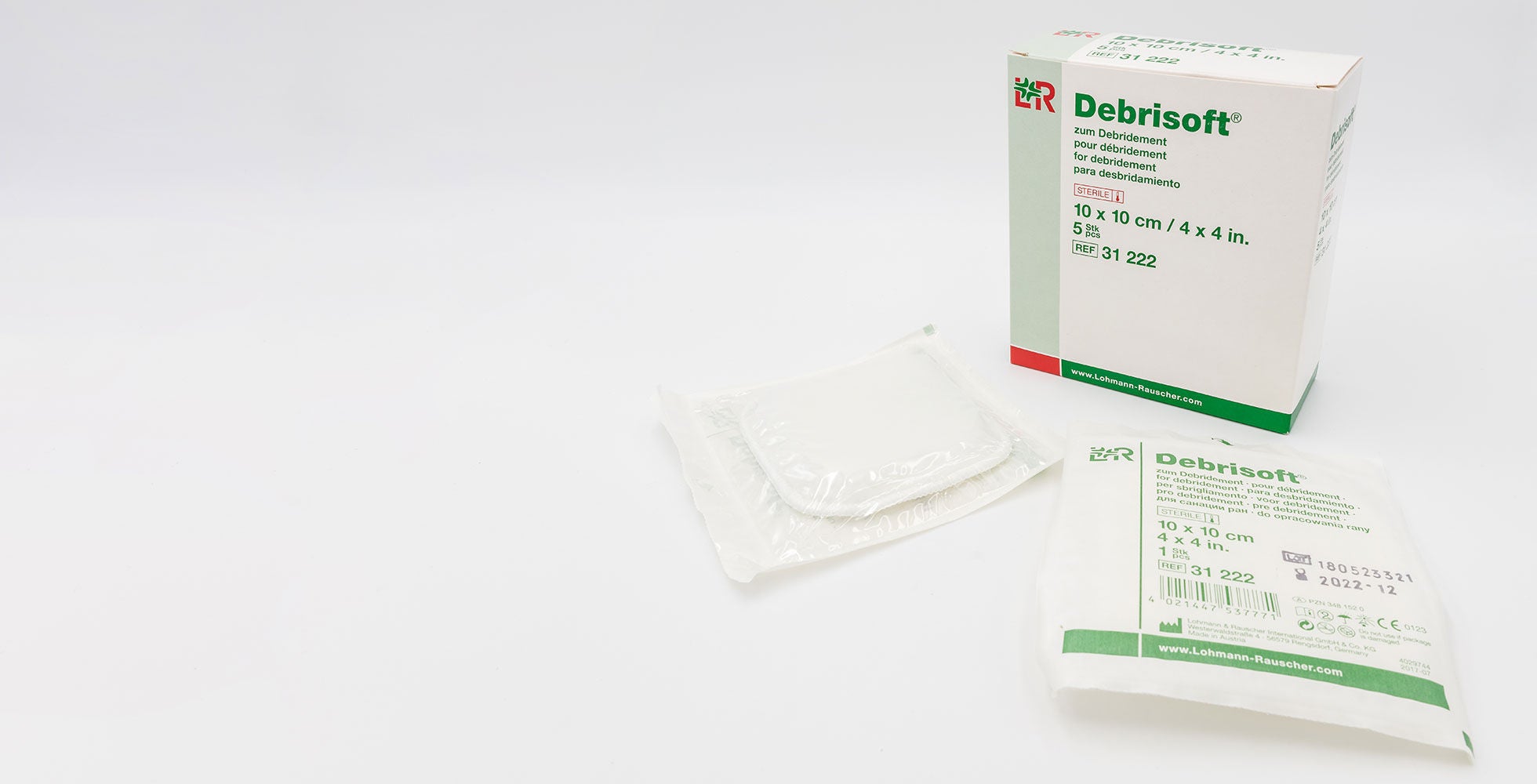
The demands placed on the packaging of medical products such as catheters, disposable products, laboratory supplies, sutures, implants, combination packs, operating theatre supplies, and diagnostics are incredibly high. The packaging must ensure that your product is delivered sterilely to the hospital, healthcare center, or patient for the point of use. It must also be opened efficiently and reliably, without the content becoming contaminated or particles becoming loose. Another important condition is that your medical products are reliably protected during transport, and their content maintains a faultless quality, even in long-term storage.
Packaging also has a vital role to play in the approval process for medical products. Even though there is often little time for product development, it is still necessary to comply with regulations and quality standards. Only in this way does the pack guarantee the highest level of security for your medical product. Sterile barrier, seal seam quality, reproducible and traceable processes, correct labelling, and much more make it essential to consult and develop your packaging solution early on.
Our machines are designed for low-bacteria and low-particle packaging of products in cleanroom environments, and they comply with the current directives (EU, EMA, FDA, DIN, ISO).
Categories
Successfully implemented packaging solutions for medical products
Our services in the area of medical products
MULTIVAC packaging solutions are built to meet your requirements and the legal requirements for reliability, efficiency, and economy. Our team of experts offers you a wide range of support services and technical assistance. Together, we develop a tailor-made packaging concept for your medical products.
Possible types of packs for medical products
- Packing in a natural atmosphere
- Modified atmosphere packaging (MAP)
- Packaging under vacuum
- Packing with skin film under vacuum
Complementary technologies
- Printing applications
- Labelling applications
- Inspection applications
- End-of-line applications
MULTIVAC solutions for the medical sector
Medical products