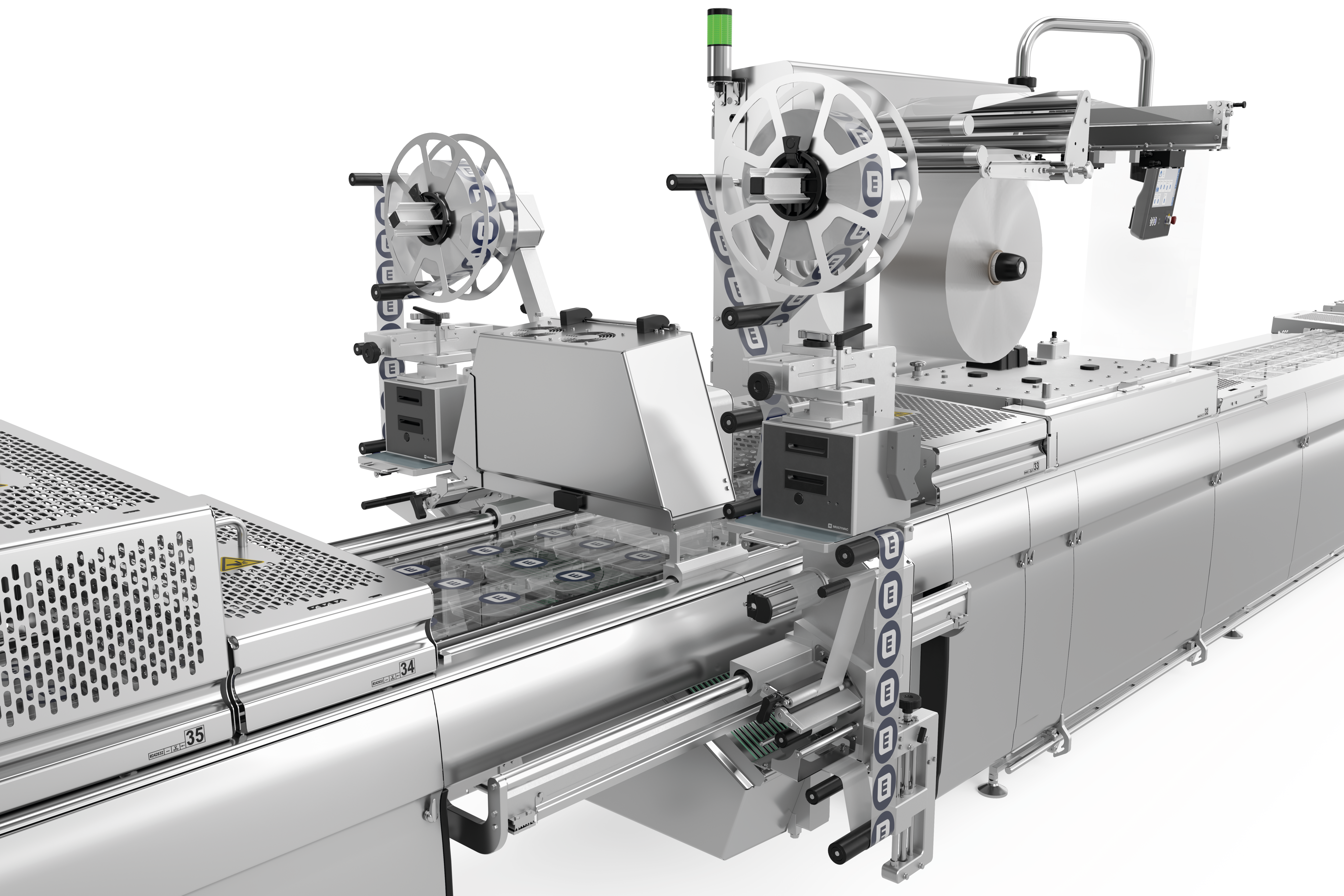
靈活、可靠,能夠保證熱成型包裝標識
針對熱成型包裝的標識,我們為您提供兩種可定制、靈活且完全集成的貼標解決方案,配有橫向貼標機和薄膜直印機。貼標機和薄膜直印機通過HMI(人機界面)集中控制。我們非常樂意針對版式和材料選擇為您提供建議。
效率和經濟性
市場需求不斷增加。公司必須緊跟發展以及市場期待,才能在未來保持自身地位。經濟上可行的投資決策必須面向未來。同樣也包括標識熱成型包裝。例如,較長的改裝時間會對設備效率產生不利影響。此外,如果單個生產線元件不作為一個具有凝聚力的單一單元工作,整個流程都將受到影響。
成功案例

Potato specialities securely packed and protected
Systematic quality assurance from the growing of the product to the cooking process and right through to packing - that is the quality claim of Peka Kroef. The Dutch producer of pre-cooked potato specialities used the opportunity presented by the replacement of some of his packaging machinery to further improve the reliability and security of his overall packaging process. In conjunction with MULTIVAC, a solution was devised, which meant a major step forward in terms of productivity, efficiency, and sustainability.
Systematic quality assurance from the growing of the product to the cooking process and right through to packing - that is the quality claim of Peka Kroef. The Dutch producer of pre-cooked potato specialities used the opportunity presented by the replacement of some of his packaging machinery to further improve the reliability and security of his overall packaging process. In conjunction with MULTIVAC, a solution was devised, which meant a major step forward in terms of productivity, efficiency, and sustainability.
了解更多

Digital printing systems fulfil challenging pack marking requirements
The UDI Directive applies to all companies, which manufacture medical products or bring these into circulation. From 25 April 2020 all products of Class III together with implants must be marked with a distinct and unique identification number, and this applies from May 2023 to products of Class IIa and IIb, as well as from 2025 to products of Class I. This distinct product identification is allocated by various bodies - these are currently GS1, HIBCC and ICCBBA. The products together with their master data and a so-called Basic UDI are registered in a new, central database (Eudamed) covering all of Europe.
The UDI Directive applies to all companies, which manufacture medical products or bring these into circulation. From 25 April 2020 all products of Class III together with implants must be marked with a distinct and unique identification number, and this applies from May 2023 to products of Class IIa and IIb, as well as from 2025 to products of Class I. This distinct product identification is allocated by various bodies - these are currently GS1, HIBCC and ICCBBA. The products together with their master data and a so-called Basic UDI are registered in a new, central database (Eudamed) covering all of Europe.
了解更多